VIP36
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| Chemical and physical data | |
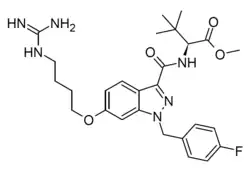
| Formula | C27H35FN6O4 |
| Molar mass | 526.613 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
VIP36 is a synthetic cannabinoid derivative, closely related to the highly potent and toxic designer drug MDMB-FUBINACA. However, unlike MDMB-FUBINACA, VIP36 has been substituted with a positively charged guanidine group which prevents it from crossing the blood-brain barrier. As a result, VIP36 is highly peripherally selective and while it produces analgesic effects in animal studies, centrally mediated side effects only became evident at a 100x higher dose.[1][2]
See also
References
- ^ Rangari VA, O'Brien ES, Powers AS, Slivicki RA, Bertels Z, Appourchaux K, et al. (April 2025). "A cryptic pocket in CB1 drives peripheral and functional selectivity". Nature. 640 (8057): 265–273. Bibcode:2025Natur.640..265R. doi:10.1038/s41586-025-08618-7. PMC 11977287. PMID 40044849.
- ^ Greig IR, Ross RA (April 2025). "Designer cannabinoids could be the key to pain relief without adverse effects". Nature. 640 (8057): 45–46. Bibcode:2025Natur.640...45G. doi:10.1038/d41586-025-00546-w. PMID 40045120.
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (modulators) |
| ||||||||||||||||||||||||||||||
| Enzyme (modulators) |
| ||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.