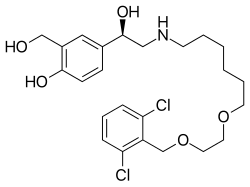
Vilanterol
 | |
| Clinical data | |
|---|---|
| License data | |
| Pregnancy category |
|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.751 |
| Chemical and physical data | |
| Formula | C24H33Cl2NO5 |
| Molar mass | 486.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vilanterol is an ultra-long-acting β2-adrenoceptor agonist which was approved in May 2013 in combination with fluticasone furoate for sale as Breo Ellipta by GlaxoSmithKline for the treatment of chronic obstructive pulmonary disease (COPD).[1][2] The combination is also approved for the treatment of asthma in Canada, Europe, Japan[3] and New Zealand.[4]
Vilanterol is available in following combinations:
- with inhaled corticosteroid fluticasone furoate—fluticasone furoate/vilanterol (trade names Breo Ellipta (U.S., NZ), Relvar Ellipta (EU, RU, JPN))
- with muscarinic antagonist umeclidinium bromide—umeclidinium bromide/vilanterol (trade name Anoro Ellipta)
- with inhaled corticosteroid fluticasone furoate and muscarinic antagonist umeclidinium bromide—fluticasone furoate/umeclidinium bromide/vilanterol (trade name Trelegy Ellipta)
See also
- Salmeterol—the long-acting β2-adrenergic receptor agonist from which vilanterol was derived.
References
- ^ "FDA approves Breo Ellipta to treat chronic obstructive pulmonary disease". Food and Drug Administration. Archived from the original on June 7, 2013. Retrieved 10 May 2013.
- ^ McKeage K (September 2014). "Fluticasone furoate/vilanterol: a review of its use in chronic obstructive pulmonary disease". Drugs. 74 (13): 1509–22. doi:10.1007/s40265-014-0269-6. PMID 25074268. S2CID 29379731.
- ^ Syed YY (March 2015). "Fluticasone furoate/vilanterol: a review of its use in patients with asthma". Drugs. 75 (4): 407–18. doi:10.1007/s40265-015-0354-5. PMID 25648266. S2CID 24563680.
- ^ Zealand (www.bka.co.nz), Site designed and developed by bka interactive ltd, Auckland, New (22 June 2019). "Fluticasone and vilanterol | Health Navigator NZ". Health Navigator New Zealand.
{{cite web}}: CS1 maint: multiple names: authors list (link)