Tulobuterol
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Inhaled, oral, transdermal patch |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.168.691 |
| Chemical and physical data | |
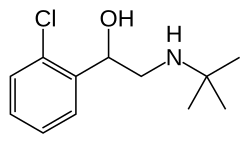
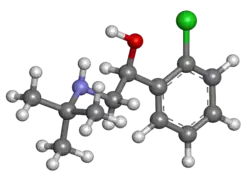
| Formula | C12H18ClNO |
| Molar mass | 227.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
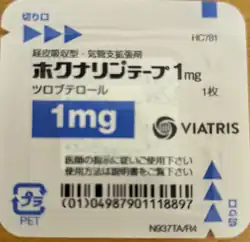
Tulobuterol (INN) is a long-acting beta2-adrenergic receptor agonist, marketed in Japan as a transdermal patch under the name Hokunalin tape (ホクナリンテープ).[1]
Currently, it is only legal in 7 countries: Japan, Germany, China, South Korea, Bangladesh, Pakistan, and Venezuela. It is available in India also.
Synthesis
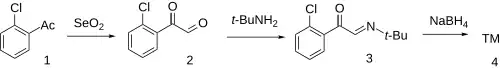
The oxidation of 2'-chloroacetophenone (1) with selenium dioxide gives o-chlorophenylglyoxal (2). Condensation with tert-butylamine gives the imine (3). Reduction with sodium borohydride completes the synthesis of tulobuterol.[2]
References
- ^ Horiguchi T, Kondo R, Miyazaki J, Fukumokto K, Torigoe H (2011). "Clinical evaluation of a transdermal therapeutic system of the beta2-agonist tulobuterol in patients with mild or moderate persistent bronchial asthma". Arzneimittel-Forschung. 54 (5): 280–5. doi:10.1055/s-0031-1296971. PMID 15212190. S2CID 9765046.
- ^ Wu CJ, Peng P, Xia LT, Liu X, Yu CX, Zheng ZB, et al. (2023). "Development of a New Process for Tulobuterol Hydrochloride". Pharmaceutical Fronts. 05: e31 – e37. doi:10.1055/s-0043-1764464.