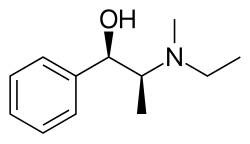
Etafedrine
 | |
| Clinical data | |
|---|---|
| Trade names | Menetyl; Nethaprin; Novedrin |
| Other names | Ethylephedrine; N-Ethylephedrine |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.218 |
| Chemical and physical data | |
| Formula | C12H19NO |
| Molar mass | 193.290 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Etafedrine (INNTooltip International Nonproprietary Name, BANTooltip British Approved Name), sold under the brand name Nethaprin among others and also known as N-ethylephedrine, is a sympathomimetic agent used as a bronchodilator to treat asthma.[2][3][4] It was previously commercially available as both the free base and as the hydrochloride salt from Sanofi-Aventis (now Sanofi) but is now no longer marketed.
Pharmacology
Unlike ephedrine and tyramine, etafedrine does not induce the release of epinephrine or norepinephrine and instead acts as a selective β2-adrenergic receptor agonist, thereby mediating its bronchodilator effects.[4][5]
See also
References
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-15.
- ^ Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 503. ISBN 978-1-4757-2085-3. Retrieved 30 August 2024.
- ^ Morton IK, Hall JM (2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Netherlands. p. 114. ISBN 978-94-011-4439-1. Retrieved 30 August 2024.
- ^ a b "Etafedrine: Uses, Interactions, Mechanism of Action". DrugBank Online. 31 December 1983. Retrieved 30 August 2024.
- ^ Lindmar R, Löffelholz K, Stieh-Koch U (1985). "On the mechanism of bronchodilatation by etafedrine". Arzneimittel-Forschung. 35 (3): 602–604. PMID 4039586.
| α1 |
| ||||
|---|---|---|---|---|---|
| α2 |
| ||||
| β |
| ||||
| |||||
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.