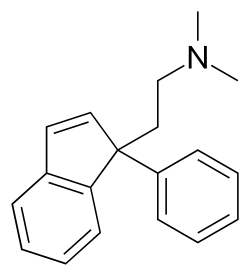
Indriline
 | |
| Clinical data | |
|---|---|
| Other names | Lu 3-083, MJ 1986. |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H21N |
| Molar mass | 263.384 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Indriline is a central nervous system stimulant with antidepressant activity and application in the treatment of gastric ulcers.
A patent using this chemical is assigned to Pharmacol.[1][2]
Synthesis
The chemical synthesis of indriline has been described:[3][4]
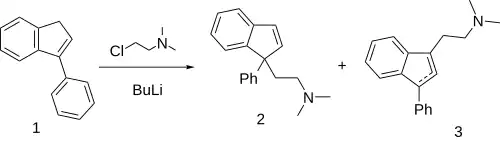
Treatment of 3-phenylindene [1961-97-3] (1) with n-butyl lithium and dimethylamino-2-choroethane gave indriline as well as some inactive isomer. Based on neighboring group participation (NGP), the reaction intermediate is presumably an aziridinium ion[4]
According to Molbase the number of routes for preparing the starting 3-phenylindene is rich. For example from dihydrochalcone.[5] A more classical synthesis is also described in a Pyrophendane patent.
References
- ^ US 3743746, Dungan K, Lish P, "Process of treating peptic ulcer with a non-anticholinergic agent", issued 1970, assigned to Mead Johnson & Co.
- ^ Buchel L, Levy J (1968). "[On the central and peripheral pharmacological properties of 1-(2 dimethylaminoethyl)-1-phenylidene]". Therapie (in French). 23 (5): 1135–46. PMID 4387506.
- ^ Dykstra SJ, Berdahl JM, Campbell KN, Combs CM, Lankin DG (May 1967). "Phenylindenes and phenylindans with antireserpine activity". Journal of Medicinal Chemistry. 10 (3): 418–428. doi:10.1021/jm00315a029. PMID 22185145.
- ^ a b US 3360435, Alexander K, Merrill LP, issued 1967, assigned to Mead Johnson & Co.
- ^ Saito S, Sato Y, Ohwada T, Shudo K (March 1994). "Friedel-Crafts-type cyclodehydration of 1,3-diphenyl-1-propanones. Kinetic evidence for the involvement of dication". Journal of the American Chemical Society. 116 (6): 2312–2317. doi:10.1021/ja00085a010.
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| Adamantanes | |
|---|---|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.