RTI-32
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H23NO2 |
| Molar mass | 273.376 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
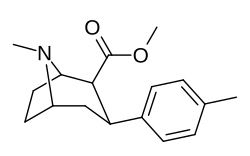
(–)-2β-Carbomethoxy-3β-(4-tolyl)tropane (RTI-4229-32, tolpane) is a phenyltropane-based cocaine analogue that has similar properties in vitro to related drugs such as RTI-31.[1][2]
| Compound | DAT | DA | NET | NA | SERT | 5HT | ED50 |
| Troparil | 23 | 49.8 | 550 | 37.2 | 178 | 173 | 0.34 |
| RTI-32 | 1.7 | 7.02 | 36 | 8.42 | 23 | 19.4 | 0.31 |
| RTI-31 | 1.1 | 3.68 | 22 | 5.86 | 4.0 | 5.0 | 0.13 |
| 3'4'-xylyl | 0.43 | unknown | 44 | unknown | 2.42 | unknown |
See also
References
- ^ Remy P, Doder M, Lees A, Turjanski N, Brooks D (June 2005). "Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system". Brain. 128 (Pt 6): 1314–22. doi:10.1093/brain/awh445. PMID 15716302.
- ^ Xu L, Izenwasser S, Katz JL, Kopajtic T, Klein-Stevens C, Zhu N, et al. (March 2002). "Synthesis and biological evaluation of 2-substituted 3beta-tolyltropane derivatives at dopamine, serotonin, and norepinephrine transporters". Journal of Medicinal Chemistry. 45 (6): 1203–10. doi:10.1021/jm010453u. PMID 11881989.
| 2-Carboxymethyl Esters | |
|---|---|
| (3,4-Disubstituted Phenyl)-tropanes | |
| Arylcarboxy | |
| Carboxyalkyl | |
| Acyl | |
| β,α Stereochemistry | |
| α,β Stereochemistry | |
| Heterocycles: 3-Substituted-isoxazol-5-yl | |
| Heterocycles: 3-Substituted-1,2,4-oxadiazole | |
| N-alkyl | |
| N-replaced (S,O,C) | |
| Irreversible | |
| Nortropanes (N-demethylated) | |
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.