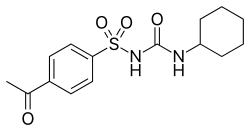
Acetohexamide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Dymelor |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a602021 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 90% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.301 |
| Chemical and physical data | |
| Formula | C15H20N2O4S |
| Molar mass | 324.40 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 188 to 190 °C (370 to 374 °F) |
| |
| |
| (verify) | |
Acetohexamide (trade name Dymelor) is a first-generation sulfonylurea medication used to treat diabetes mellitus type 2, particularly in people whose diabetes cannot be controlled by diet alone.[1]
Mechanism of action
Acetohexamide binds to an ATP-sensitive K+ (KATP) channel on the cell membrane of pancreatic beta cells. This inhibits the outflux of potassium, which causes the membrane potential to become more positive. This depolarization in turn opens voltage-gated calcium channels. The rise in intracellular calcium leads to increased fusion of insulin granulae with the cell membrane, and therefore increased secretion of insulin.[2]
Risks
Sulfonylureas, especially first-generation sulfonylureas such as Acetohexamide, can cause severe hypoglycemia and increase the risk of adverse cardiovascular events. [3][4]
References
- ^ Montgomery DA (October 1964). "Current Therapeutics. CCII. Acetohexamide". The Practitioner. 193: 555–60. PMID 14216839.
- ^ "Acetohexamide". DrugBank.
- ^ "www.accessdata.fda.gov" (PDF). Archived from the original (PDF) on January 2, 2015.
- ^ "Acetohexamide". Medline Plus. Archived from the original on 11 September 2005.