Cyclopentobarbital
 | |
| Clinical data | |
|---|---|
| Other names | Allylpental, Cyclopental, 5-Allyl-5-Δ2-Cyclopentenyl Barbituric Acid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.891 |
| Chemical and physical data | |
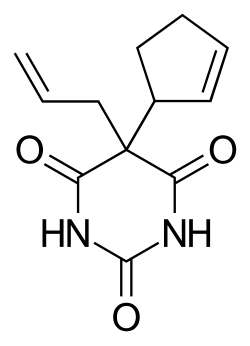
| Formula | C12H14N2O3 |
| Molar mass | 234.255 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cyclopentobarbital sodium (Cyclopal, Dormisan) is a barbiturate derivative invented in the 1940s.[1] It has sedative and anticonvulsant properties, and was used primarily as an anaesthetic in veterinary medicine.[2] Cyclopal is considered similar in effects to phenobarbital but lasts almost three times as long, and is considered a long-acting barbiturate with a fairly slow onset of action.
See also
References
- ^ Martin JR, Godel T, Hunkeler W, Jenck F, Moreau JL, Sleight AJ, Widmer U (December 2000). "Psychopharmacological agents.". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.1619250313011820.a01. ISBN 0471238961.
- ^ Vander Brook MJ, Cartland GF (1944). "A Pharmacologic Study of 5-Allyl-5-Cyclopentenyl Barbituric Acid (Cyclopal)". Journal of Pharmacology and Experimental Therapeutics. 80 (2): 119–125. CiteSeerX 10.1.1.983.6071.