SL-164
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
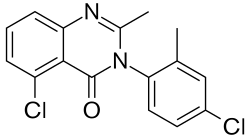
| Formula | C16H12Cl2N2O |
| Molar mass | 319.19 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
SL-164, also known as dicloqualone or DCQ, is an analogue of methaqualone developed in the late 1960s by a team at Sumitomo.[1] SL-164 has similar sedative, hypnotic[2] and properties to the parent compound, but was never marketed for clinical use, due to higher risk of convulsions. Like other 4-substituted analogues, such as methylmethaqualone, SL-164 may cause seizures.[3]
References
- ^ US 3651230, "Compositions And Methods For Tranquilizing With Substituted 3-Phenyl-4-Quinazolinone Derivatives"
- ^ Saito C, Sakai S, Yukawa Y, Yamamoto H, Takagi H (December 1969). "Pharmacological studies on 2-methyl-3(2'-methyl-4'-chlorophenyl)-5-chloro-4[H)-quinazolinone (SL-164)". Arzneimittel-Forschung. 19 (12): 1945–9. PMID 4985336.
- ^ Boltze KH, Dell HD, Lehwald H, Lorenz D, Rueberg-Schweer M (August 1963). "[Substituted 4-Quinazolinone Derivatives As Hypnotics]". Arzneimittel-Forschung (in German). 13: 688–701. PMID 14085923.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.