ROD-188
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
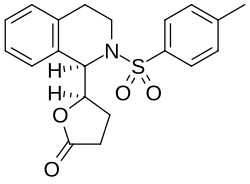
| Formula | C20H21NO4S |
| Molar mass | 371.45 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
ROD-188 is a sedative drug that was structurally derived from the GABAA antagonist bicuculline by a team at Roche.[1] Unlike bicuculline, ROD-188 acts as an agonist at GABAA receptors, being a positive allosteric modulator acting at a novel binding site distinct from those of benzodiazepines, barbiturates or muscimol, with its strongest effect produced at the α6β2γ2 subtype of the GABAA receptor.[2] ROD-188 is one of a number of related compounds acting at this novel modulatory site, some of which also act at benzodiazepine receptors.[3][4]
See also
References
- ^ US 6649626, "N-substituted 1-(lactone) isoquinolones for treating nervous disorders"
- ^ Thomet U, Baur R, Razet R, Dodd RH, Furtmüller R, Sieghart W, Sigel E (October 2000). "A novel positive allosteric modulator of the GABA(A) receptor: the action of (+)-ROD188". British Journal of Pharmacology. 131 (4): 843–850. doi:10.1038/sj.bjp.0703558. PMC 1572371. PMID 11030736.
- ^ Sigel E, Baur R, Furtmueller R, Razet R, Dodd RH, Sieghart W (June 2001). "Differential cross talk of ROD compounds with the benzodiazepine binding site". Molecular Pharmacology. 59 (6): 1470–1477. doi:10.1124/mol.59.6.1470. PMID 11353808.
- ^ Ramerstorfer J, Furtmüller R, Sarto-Jackson I, Varagic Z, Sieghart W, Ernst M (January 2011). "The GABAA receptor α+β-interface: a novel target for subtype selective drugs". The Journal of Neuroscience. 31 (3): 870–877. doi:10.1523/JNEUROSCI.5012-10.2011. PMC 3182524. PMID 21248110. S2CID 1989995.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
| Ionotropic |
| ||||
|---|---|---|---|---|---|
| Metabotropic |
| ||||
| |||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.