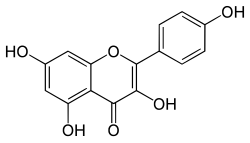
Kaempferol
Names
IUPAC name
3,4′,5,7-Tetrahydroxyflavone
Systematic IUPAC name
3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H -1-benzopyran-4-one
Other names
Kaempherol; Robigenin; Pelargidenolon; Rhamnolutein; Rhamnolutin; Populnetin; Trifolitin; Kempferol; Swartziol
Identifiers
ChEBI
ChEMBL
ChemSpider
DrugBank
ECHA InfoCard 100.007.535
EC Number
KEGG
UNII
InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
Key: IYRMWMYZSQPJKC-UHFFFAOYSA-N
O=c1c(O)c(-c2ccc(O)cc2)oc2cc(O)cc(O)c12
Properties
C15 H10 O6
Molar mass
286.23 g/mol
Density
1.688 g/mL
Melting point
276–278 °C
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Kaempferol (3,4′,5,7-tetrahydroxyflavone) is a natural flavonol , a type of flavonoid , found in a variety of plants and plant-derived foods including kale , beans , tea , spinach , and broccoli .[ 1] [ 2] ethanol , ethers , and DMSO . Kaempferol is named for 17th-century German naturalist Engelbert Kaempfer .[ 3]
Natural occurrence
Kaempferol is a secondary metabolite found in many plants, plant-derived foods, and traditional medicines.[ 4]
In plants and food
Kaempferol is common in Pteridophyta , Pinophyta , and Angiospermae . Within Pteridophyta and Pinophyta, kaempferol has been found in diverse families. Kaempferol has also been identified in Dicotyledons and Monocotyledons of Angiosperms.[ 4] [ 5] apples ,[ 6] grapes ,[ 6] tomatoes ,[ 6] green tea ,[ 6] potatoes ,[ 5] onions ,[ 4] broccoli ,[ 4] Brussels sprouts ,[ 4] squash ,[ 4] cucumbers ,[ 4] lettuce ,[ 4] green beans ,[ 4] peaches ,[ 4] blackberries ,[ 4] raspberries ,[ 4] spinach .[ 4] Aloe vera [ 4] Coccinia grandis [ 4] Cuscuta chinensis [ 7] Euphorbia pekinensis [ 4] Glycine max [ 4] Hypericum perforatum [ 4] Pinus sylvestris [ 8] Moringa oleifera [ 9] Rosmarinus officinalis [ 4] Sambucus nigra [ 4] Toona sinensis [ 4] Ilex [ 4] endive .[ 10]
Foods
Kaempferol
(mg/100 g)
capers , raw
259[ 11]
saffron
205[ 11]
capers , canned
131[ 11]
arugula , raw
59[ 11]
kale , raw
47[ 11]
mustard greens , raw
38[ 11]
ginger
34[ 11]
common bean , raw
26[ 11]
chinese cabbage , raw
23[ 11]
dill , fresh
13[ 11]
garden cress , raw
13[ 11]
chive , raw
10[ 11]
dock , raw
10[ 11]
endive , raw
10[ 11]
collard , raw
9[ 11]
broccoli , raw
8[ 11]
fennel leaves
7[ 11]
goji berry , dried
6[ 11]
drumstick leaves, raw
6[ 11]
chard , raw
4[ 11]
Biosynthesis
The biosynthesis of kaempferol occurs in four major steps:[ 4]
The amino acid phenylalanine is formed from the Shikimate pathway , which is the pathway that plants use in order to make aromatic amino acids. This pathway is located in the plant plastid , and is the entry to the biosynthesis of phenylpropanoids.[ 12]
The phenylpropanoid pathway is the pathway that converts phenylalanine into tetrahydroxychalcone. Flavonols, including kaempferol, are products of this pathway.[ 13]
Notes
^ Holland TM, Agarwal P, Wang Y, Leurgans SE, Bennett DA, Booth SL, Morris MC (2020-01-29). "Dietary flavonols and risk of Alzheimer dementia" . Neurology . 94 (16): e1749 – e1756 . doi :10.1212/WNL.0000000000008981 . ISSN 0028-3878 . PMC 7282875 PMID 31996451 . ^ Bertolucci V, Ninomiya AF, Longato GB, Kaneko LO, Nonose N, Scariot PP, Messias LH (2025-01-12). "Bioactive Compounds from Propolis on Bone Homeostasis: A Narrative Review" . Antioxidants . 14 (1): 81. doi :10.3390/antiox14010081 ISSN 2076-3921 . PMC 11762496 PMID 39857415 . ^ Kaempferol at Merriam-Webster .com; retrieved October 20, 2017^ a b c d e f g h i j k l m n o p q r s t u v w Calderón Montaño JM, Burgos Morón E, Pérez Guerrero C, López Lázaro M (April 2011). "A review on the dietary flavonoid kaempferol". Mini Reviews in Medicinal Chemistry . 11 (4): 298– 344. doi :10.2174/138955711795305335 . PMID 21428901 . ^ a b Liu RH (May 2013). "Health-promoting components of fruits and vegetables in the diet" . Advances in Nutrition . 4 (3): 384S – 392S . doi :10.3945/an.112.003517 . PMC 3650511 PMID 23674808 . ^ a b c d Kim SH, Choi KC (December 2013). "Anti-cancer Effect and Underlying Mechanism(s) of Kaempferol, a Phytoestrogen, on the Regulation of Apoptosis in Diverse Cancer Cell Models" . Toxicological Research . 29 (4): 229– 234. Bibcode :2013ToxRe..29..229K . doi :10.5487/TR.2013.29.4.229 . PMC 3936174 PMID 24578792 . ^ Donnapee S, Li J, Yang X, Ge AH, Donkor PO, Gao XM, Chang YX (November 2014). "Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine". Journal of Ethnopharmacology . 157 (C): 292– 308. doi :10.1016/j.jep.2014.09.032 . PMID 25281912 . ^ de la Luz Cádiz-Gurrea M, Fernández-Arroyo S, Segura-Carretero A (November 2014). "Pine bark and green tea concentrated extracts: antioxidant activity and comprehensive characterization of bioactive compounds by HPLC-ESI-QTOF-MS" . International Journal of Molecular Sciences . 15 (11): 20382– 20402. doi :10.3390/ijms151120382 PMC 4264173 PMID 25383680 . ^ Anwar F, Latif S, Ashraf M, Gilani AH (January 2007). "Moringa oleifera: a food plant with multiple medicinal uses" . Phytotherapy Research . 21 (1): 17– 25. doi :10.1002/ptr.2023 PMID 17089328 . ^ DuPont MS, Day AJ, Bennett RN, Mellon FA, Kroon PA (June 2004). "Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans". European Journal of Clinical Nutrition . 58 (6): 947– 954. doi :10.1038/sj.ejcn.1601916 . PMID 15164116 . S2CID 25720976 . ^ a b c d e f g h i j k l m n o p q r s t "USDA Database for the Flavonoid Content of Selected Foods, Release 3" (PDF) . U.S. Department of Agriculture. 2011.^ Vogt T (January 2010). "Phenylpropanoid biosynthesis" . Molecular Plant . 3 (1): 2– 20. doi :10.1093/mp/ssp106 PMID 20035037 . ^ Flamini R, Mattivi F, De Rosso M, Arapitsas P, Bavaresco L (September 2013). "Advanced knowledge of three important classes of grape phenolics: anthocyanins, stilbenes and flavonols" . International Journal of Molecular Sciences . 14 (10): 19651– 19669. doi :10.3390/ijms141019651 PMC 3821578 PMID 24084717 .
External links
Food antioxidants Fuel antioxidants Measurements
Flavonols and their conjugates
Backbone
Flavonols
Aglycones Conjugates
Glycosides of herbacetin Glycosides of
Afzelin (Kaempferol 3-rhamnoside)Astragalin (kaempferol 3-O-glucoside)Kaempferitrin (kaempferol 3,7-dirhamnoside)Juglanin (Kaempferol 3-O-arabinoside)Kaempferol 3-alpha-L-arabinopyranoside
Kaempferol 3-alpha-D-arabinopyranoside
Kaempferol 7-alpha-L-arabinoside
Kaempferol 7-O-glucoside Kaempferol 3-lathyroside
Kaempferol 4'-rhamnoside
Kaempferol 5-rhamnoside
Kaempferol 7-rhamnoside
Kaempferol 7-O-alpha-L-rhamnofuranoside
Kaempferol 3-xyloside
Kaempferol 7-xyloside
Robinin (kaempferol-3-O-robinoside-7-O-rhamnoside)Kaempferol 3-O-rutinoside Sophoraflavonoloside (Kaempferol 3-O-sophoroside)
Trifolin (Kaempferol 3-O-beta-D-galactoside) Glycosides of myricetin Conjugates of quercetin
O -Methylated flavonols
Aglycones Glycosides
of isorhamnetin
Narcissin (Isorhamnetin 3-O-rutinoside)
Isorhamnetin 3-O-glucoside
Tamarixetin 7-rutinoside other
Azalein (Azaleatin 3-O-α-L-rhamnoside)Centaurein (Centaureidin 7-O-glucoside)
Eupalin (Eupalitin 3-0-rhamnoside)Eupatolin (Eupatolitin 3-O-rhamnoside)Jacein (Jaceidin 7-O-glucoside)
Patulitrin (Patuletin 7-O-glucoside
Xanthorhamnin (Rhamnetin glycoside)
Derivative flavonols
Aglycones
Noricaritin
Dihydronoricaritin Glycosides
Pyranoflavonols
Furanoflavonols
Semisynthetic
Receptor (ligands )
CB1 Tooltip Cannabinoid receptor type 1
Agonists(abridged, Inverse agonists Antagonists
CB2 Tooltip Cannabinoid receptor type 2
Agonists
2-AG 2-AGE (noladin ether) 3,3'-Diindolylmethane 4-O-Methylhonokiol α-Amyrin · β-Amyrin A-796,260 A-834,735 A-836,339 AM-1172
AM-1221 AM-1235 AM-1241 AM-2232 Anandamide AZ-11713908 Cannabinol Caryophyllene CB-13 CBS-0550 CP 55,940 GW-405,833 (L-768,242) GW-842,166X HU-308 JTE 7-31 JWH-007 JWH-015 JWH-018 JWH-73
JWH-133 L-759,633 L-759,656 Lenabasum (anabasum) Magnolol MDA-19 Nabitan NADA Olorinab (APD-371) PF-03550096 S-444,823 SER-601 Serinolamide A UR-144 Tedalinab THC (dronabinol) THCV Tetrahydromagnolol
Virodhamine Antagonists
NAGly GPR18 )
GPR55
GPR119
Transporter (modulators )
eCBTs Tooltip Endocannabinoid transporter
Enzyme (modulators)
FAAH Tooltip Fatty acid amide hydrolase MAGL ABHD6
Inhibitors: JZP-169JZP-430
KT182
KT185
KT195
KT203
LEI-106
ML294
ML295
ML296
UCM710
WWL-70 ABHD12
Others
Others: 2-PG (directly potentiates activity of 2-AG at CB1 receptor) ARN-272 (FAAH-like anandamide transporter inhibitor)
See also
Receptor/signaling modulators Cannabinoids (cannabinoids by structure)
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel
3β,5α-Dihydrolevonorgestrel
3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , , liquiritigenin , mirificin , myricetin , naringenin , penduletin, pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown
See also
Receptor/signaling modulators
Estrogens and antiestrogens
Androgen receptor modulators
Progesterone receptor modulators
List of estrogens
PR Tooltip Progesterone receptor
Agonists
Testosterone derivatives: Progestins: 6,6-Difluoronorethisterone 6,6-Difluoronorethisterone acetate 17α-Allyl-19-nortestosterone Allylestrenol Altrenogest Chloroethynylnorgestrel Cingestol Danazol Desogestrel Dienogest Ethinylandrostenediol
Ethisterone Ethynerone Etonogestrel Etynodiol Etynodiol diacetate Gestodene Gestrinone Levonorgestrel Levonorgestrel esters (e.g., levonorgestrel butanoate )Lynestrenol Lynestrenol phenylpropionate Metynodiol Metynodiol diacetate Norelgestromin Norethisterone (norethindrone) Norethisterone esters (e.g., norethisterone acetate , norethisterone enanthate )Noretynodrel Norgesterone Norgestimate Norgestrel Norgestrienone Norvinisterone Oxendolone Quingestanol Quingestanol acetate Tibolone Tigestol Tosagestin ; Anabolic–androgenic steroids: 11β-Methyl-19-nortestosterone 11β-Methyl-19-nortestosterone dodecylcarbonate 19-Nor-5-androstenediol 19-Nor-5-androstenedione 19-Nordehydroepiandrosterone Bolandiol Bolandiol dipropionate Bolandione Dimethisterone Dienedione Dienolone Dimethandrolone Dimethandrolone buciclate Dimethandrolone dodecylcarbonate Dimethandrolone undecanoate Dimethyldienolone Dimethyltrienolone Ethyldienolone Ethylestrenol (ethylnandrol) Methyldienolone Metribolone (R-1881) Methoxydienone (methoxygonadiene) Mibolerone Nandrolone Nandrolone esters (e.g., nandrolone decanoate , nandrolone phenylpropionate )Norethandrolone Normethandrone (methylestrenolone, normethandrolone, normethisterone) RU-2309 Tetrahydrogestrinone Trenbolone (trienolone) Trenbolone esters (e.g., trenbolone acetate , trenbolone enanthate )Trendione Trestolone Trestolone acetate MixedSPRMs Tooltip Selective progesterone receptor modulators ) Antagonists
mPR Tooltip Membrane progesterone receptor PAQR Tooltip Progestin and adipoQ receptor )
See also
Receptor/signaling modulators
Progestogens and antiprogestogens
Androgen receptor modulators
Estrogen receptor modulators
List of progestogens
 Media related to Kaempferol at Wikimedia Commons
Media related to Kaempferol at Wikimedia Commons