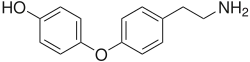
Thyronamine
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-[4-(2-Aminoethyl)phenoxy]phenol | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider |
|
| MeSH | thyronamine |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H15NO2 | |
| Molar mass | 229.279 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Thyronamine refers both to a molecule, and to derivatives of that molecule: a family of decarboxylated and deiodinated metabolites of the thyroid hormones thyroxine (T4) and 3,5,3'-triiodothyronine (T3).
Types
The group includes:
- Thyronamine (T0AM)
- 3-Iodothyronamine (T1AM), which is the most notable one as it is a trace amine found in the nervous system. It is a possible candidate for the natural ligand of the trace amine-associated receptor TAAR1 (TAR1), an intracellular G protein-coupled receptor[1]
- 3,5-Diiodothyronamine (T2AM)
- 3,5,3'-Triiodothyronamine (T3AM)
See also
References
- ^ Piehl S, Hoefig CS, Scanlan TS, Köhrle J (2011). "Thyronamines - Past, Present, and Future". Endocrine Reviews. 32 (1): 64–80. doi:10.1210/er.2009-0040. PMID 20880963.
| Tyrosine / iodotyrosine | |
|---|---|
| Thyronine / iodothyronine | |
| / iodothyronamine | |
| Iodothyroacetate / iodothyroacetic acid | |
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.