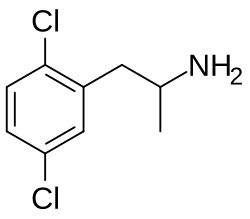
2,4-Dichloroamphetamine
 | |
| Clinical data | |
|---|---|
| Other names | 2,4-DCA |
| Drug class | Psychostimulant; Serotonergic neurotoxin; Monoamine oxidase inhibitor |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C9H11Cl2N |
| Molar mass | 204.09 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
2,4-Dichloroamphetamine (2,4-DCA) is a psychostimulant of the amphetamine family and a potent serotonergic neurotoxin related to para-chloroamphetamine (PCA; 4-chloroamphetamine).[1][2] It is also a potent monoamine oxidase inhibitor.[1][3][2]
See also
- 3-Chloroamphetamine (3-CA)
- 3,4-Dichloroamphetamine (3,4-DCA)
References
- ^ a b Biel JH, Bopp BA (1978). "Amphetamines: Structure-Activity Relationships". In Iversen L, Snyder SH, Iversen SD (eds.). Stimulants. Boston, MA: Springer US. p. 1–39. doi:10.1007/978-1-4757-0510-2_1. ISBN 978-1-4757-0512-6.
- ^ a b Fuller RW, Snoddy HD, Roush BW, Molloy BB (January 1973). "Further structure-activity studies on the lowering of brain 5-hydroxyindoles by 4-chloramphetamine". Neuropharmacology. 12 (1). Elsevier BV: 33–42. doi:10.1016/0028-3908(73)90129-9. PMID 4687274.
- ^ Conde S, Madroñero R, Fernández-Tomé MP, del Río J (September 1978). "Effects of thiophene analogues of chloroamphetamines on central serotonergic mechanisms". Journal of Medicinal Chemistry. 21 (9). American Chemical Society (ACS): 978–981. doi:10.1021/jm00207a024. PMID 722762.
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.