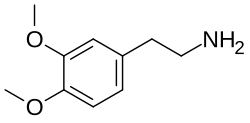
3,4-Dimethoxyphenethylamine
Names
Preferred IUPAC name
2-(3,4-Dimethoxyphenyl)ethan-1-amine
Other names
O ,O -Dimethyldopamine; Homoveratrylamine
Identifiers
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.003.979
UNII
InChI=1S/C10H15NO2/c1-12-9-4-3-8(5-6-11)7-10(9)13-2/h3-4,7H,5-6,11H2,1-2H3
Y Key: ANOUKFYBOAKOIR-UHFFFAOYSA-N
Y InChI=1/C10H15NO2/c1-12-9-4-3-8(5-6-11)7-10(9)13-2/h3-4,7H,5-6,11H2,1-2H3
Key: ANOUKFYBOAKOIR-UHFFFAOYAB
Properties
C10 H15 NO2
Molar mass
181.23 g/mol
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
3,4-Dimethoxyphenethylamine (DMPEA ), also known as O ,O -dimethyldopaminechemical compound of the phenethylamine class. It is an analogue of the major human neurotransmitter dopamine where the 3- and 4-position hydroxy groups have been replaced with methoxy groups. It is also closely related to mescaline which is 3,4,5-trimethoxyphenethylamine and to 3,4-dimethoxyamphetamine (3,4-DMA).
Pharmacology
DMPEA shows weak affinity for serotonin receptors .[ 1] head-twitch response , a behavioral proxy of serotonergic psychedelic effects, in rodents.[ 2] Alexander Shulgin , it was inactive in humans at doses of 1,000 mg orally and 10 mg intravenously .[ 3]
DMPEA has some activity as a monoamine oxidase inhibitor .[ 4]
The elimination half-life of DMPEA is said to be less than 1 hour, indicating rapid and extensive metabolism and inactivation.[ 5] [ 6]
Chemistry
One of the earliest syntheses of DMPEA (then referred to as "homoveratrylamine") was that of Pictet and Finkelstein, who made it in a multi-step sequence starting from vanillin .[ 7] [ 8]
3,4-Dimethoxybenzaldehyde (veratraldehyde) → 3,4-Dimethoxycinnamic acid → 3,4-Dimethoxyphenylpropionic acid → 3,4-Dimethoxyphenylpropionamide → 3,4-DimethoxyphenethylamineA much shorter synthesis is given by Shulgin and Shulgin:[ 9] [ 10]
Derivatives
A known use was in the synthesis of bevantolol .
Natural occurrence
DMPEA occurs naturally along with mescaline in various species of cacti such as San Pedro and Peruvian Torch .[ 11] [ 12] [ 13]
See also
References
^ Glennon RA, Liebowitz SM, Anderson GM (March 1980). "Serotonin receptor affinities of psychoactive phenalkylamine analogues". J Med Chem . 23 (3): 294– 299. doi :10.1021/jm00177a017 . PMID 7365744 . ^ Corne SJ, Pickering RW, Warner BT (February 1963). "A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine" . Br J Pharmacol Chemother . 20 (1): 106– 120. doi :10.1111/j.1476-5381.1963.tb01302.x . PMC 1703746 PMID 14023050 . ^ Shulgin AT (2003). "Basic Pharmacology and Effects" . In Laing RR (ed.). Hallucinogens: A Forensic Drug Handbook 67– 137. ISBN 978-0-12-433951-4 . Retrieved 1 February 2025 . ^ Keller WJ; Ferguson GG (July 1977). "Effects of 3,4-dimethoxyphenethylamine derivatives on monoamine oxidase". Journal of Pharmaceutical Sciences . 66 (7): 1048– 50. doi :10.1002/jps.2600660741 . PMID 886445 . ^ Nichols DE (August 1981). "Structure-activity relationships of phenethylamine hallucinogens" . J Pharm Sci . 70 (8): 839– 849. doi :10.1002/jps.2600700802 . PMID 7031221 . ^ Schweitzer JW, Friedhoff AJ (March 1968). "The metabolism of dimethoxyphenethylamine, a compound found in the urine of schizophrenics". Am J Psychiatry . 124 (9): 1249– 1253. doi :10.1176/ajp.124.9.1249 . PMID 5637912 . ^ A. Pictet and M. Finkelstein (1909). "Synthese des Laudanosins." Ber. 42 1979-1989.
^ J. S. Buck and W. H. Perkin (1924). "CCXVIII. Ψ-epiBerberine." J. Chem. Soc., Trans. 125 1675-1686.
^ A. Shulgin and A. Shulgin (1991). "PiHKAL A Chemical Love Story", pp. 614-616, Transform Press, Berkeley. ISBN 0-9630096-0-5
^ "Erowid Online Books : "PIHKAL" - #60 DMPEA" .^ Lundström J (December 1970). "Biosynthesis of mescaline and 3,4-dimethoxyphenethylamine in Trichocereus pachanoi Br&R". Acta Pharmaceutica Suecica . 7 (6): 651– 66. PMID 5511715 . ^ Pummangura S; Nichols DE; McLaughlin JL (October 1977). "Cactus alkaloids XXXIII: beta-phenethylamines from the Guatemalan cactus Pilosocereus maxonii". Journal of Pharmaceutical Sciences . 66 (10): 1485– 7. doi :10.1002/jps.2600661037 . PMID 925910 . ^ Pardanani JH; McLaughlin JL; Kondrat RW; Cooks RG (1977). "Cactus alkaloids. XXXVI. Mescaline and related compounds from Trichocereus peruvianus". Lloydia . 40 (6): 585– 90. PMID 600028 .
External links
No ring subs. 4-Hydroxytryptamines 5-Hydroxytryptamines 5-Methoxytryptamines Other ring subs.
2,N ,N -TMT 4,N ,N -TMT
5-Bromo-DMT 5-Chloro-DMT 5-Fluoro-DMT 5-N ,N -TMT 7,N ,N -TMT 5-MeO-2,N ,N -TMT 5-MeO-4,N ,N -TMT
6-Fluoro-DMT Bretisilocin (GM-2505; 5-fluoro-MET) α-Alkyltryptamines
5-Methoxy-α-alkyltryptamines: 5-MeO-AET α,N ,N -TMT (α-Me-DMT; Alpha-N) 5-MeO-AMT (α,O -DMS; Alpha-O) α,N ,O -TMS (5-MeO-α,N -DMT) α,N ,N ,O -TeMS (5-MeO-α,N ,N -TMT) Others
Ergolines /lysergamides (e.g., LSD )β-Carbolines and Harmala alkaloidsharmine , harmaline , 6-methoxyharmalan )Iboga alkaloids18-MAC , 18-MC , coronaridine , ibogaine , ibogamine , ME-18-MC , noribogaine , tabernanthine , voacangine )Ibogalogs (e.g., ibogainalog )O -MethylnordehydrobufoteninePartial ergolines (e.g., NDTDI , RU-28306 , CT-5252 )Piperidinylethylindoles (e.g., pip-T )Pyrrolidinylethylindoles (e.g., pyr-T , 5-MeO-pyr-T )Pyrrolidinylmethylindoles (e.g., MPMI , 4-HO-MPMI (lucigenol) , 5-MeO-MPMI )
Benzofurans (e.g., 5-MeO-DiBF , dimemebfe (5-MeO-BFE) , mebfap )Benzothiophenes (e.g., 3-APBT )Indazolethylamines (e.g., AL-38022A , O -methyl-AL-34662Indenylethylamines (e.g., C-DMT )Isotryptamines (e.g., 6-MeO-isoDMT , Ro60-0175 )MYCO-005 Quinolinylethylamines (e.g., mefloquine )
Others: 2C-G-x (e.g., 2C-G-3 , 2C-G-5 )β-Keto-2C-B (βk-2C-B) β-Keto-2C-I (βk-2C-I)
β-Methyl-2C-B (BMB) BOB , BOD , BOH-2C-B )HOT-2 , HOT-7 , HOT-17 )N -Ethyl-2C-B2CD-5-ETO , 2CE-5-ETO, 2CE-5iPrO , 2CT2-5-ETO, ASR-2001 (2CB-5PrO) ) Others
2-TOET 2-TOM 25B-NAcPip 4-HA 5-TOET 5-TOM Benzofurans (e.g., 5-APB , 5-APDB , 6-APB , 6-APDB , F , F-2 , F-22 )Benzothiophenes (e.g., 5-APBT , 6-APBT )CT-5172 DMAs (e.g., 2,4-DMA , 3,4-DMA )Fenfluramine MMA (3-MeO-4-MA) Norfenfluramine 25D-NM-NDEAOP , DOB-NDEPA , DOI-NDEPA , DOM-NDEPA, DOTFM-NDEPA , M-NDEPA, TMA-2-NDEPA)PMA (4-MA) TMA-3 , TMA-4 , TMA-5 )TOMSO ZDCM-04
1-Aminomethylindanes (e.g., 2CB-Ind , jimscaline )2-Aminoindanes (e.g., DOM-AI )3-Phenylpiperidines (e.g., LPH-5 , LPH-48 )Benzazepines (e.g., lorcaserin )Benzocyclobutenes (e.g., 2CBCB-NBOMe , TCB-2 , tomscaline )Benzoxepins (e.g., BBOX, IBOX, TFMBOX )DMBMPP (juncosamine) Ergolines /lysergamides (e.g., LSD )Glaucine Partial ergolines (e.g., NDTDI , DEIMDHPCA , DEMPDHPCA , DEMTMPDHPCA , DEMNDHPCA )Phenylcyclopropylamines (e.g., DMCPA , TMT )Phenyloxazolamines (aminorexes ) (e.g., 2C-B-aminorex )Pyridopyrroloquinoxalines (e.g., IHCH-7113 )Z3517967757 ZC-B
Others
Arylpiperazines (e.g., 2C-B-PP , 2-NP , mCPP , MK-212 , ORG-12962 , pCPP , pFPP , quipazine , TFMPP )Dihydrobenzoxazines (e.g., efavirenz )Phenoxyethylamines (e.g., CT-4719 , ORG-37684 )Pyridopyrroloquinoxalines (e.g., IHCH-7113 )Quinazolinylethylamines (e.g., RH-34 ) Natural sources
Tryptamines: Acacia spp.Acacia acuminata Acacia confusa Ayahuasca and vinho de Jurema (e.g., Psychotria viridis (chacruna)Dipolopterys cabrerana (chaliponga, chacruna)Mimosa tenuiflora (Mimosa hostilis ; jurema)Brosimum Brosimum acutifolium (takini)Hallucinogenic snuffs (e.g., Anadenanthera peregrina (yopo, jopo, cohoba, parica, ebene)Anadenanthera colubrina (vilca, cebil)Incilius alvarius (Bufo alvarius ; Colorado River toad, Sonoran Desert toad; bufo)Psilocybin-containing mushrooms (magic mushrooms, shrooms) (e.g., Psilocybe cubensis Psilocybe mexicana (teonanacatl)Lysergamides: Achnatherum robustum (sleepy grass)Epichloë spp.Ergot (Claviceps ) (e.g., Claviceps purpurea Claviceps paspali Morning glory (Convolvulaceae) seeds (e.g., Ipomoea tricolor (tlitliltzin, badoh negro; Ipomoea violacea )Ipomoea corymbosa (coaxihuitl, ololiúqui; Rivea Corymbosa , Turbina Corymbosa )Argyreia nervosa (Hawaiian baby woodrose; HBWR)Periglandula spp.Periglandula ipomoeae Periglandula clandestina
See also: HallucinogensEntactogens Tryptamines Phenethylamines Ergolines and lysergamides Serotonin receptor modulators
Phenethylamines Amphetamines Phentermines Cathinones Phenylisobutylamines (and further-extended) Catecholamines (and close relatives) Cyclized
Phenylalkylpyrrolidines 2-Benzylpiperidines (phenidates ) Phenylmorpholines (phenmetrazines) Phenyloxazolamines (aminorexes) Isoquinolines andtetrahydroisoquinolines 2-Aminoindanes 2-Aminotetralins Others / unsorted
1-Aminomethylindanes (e.g., 2CB-Ind , AMMI , bromojimscaline, jimscaline )2-ADN 2-Benzhydrylpyrrolidine
2C-B-5-hemiFLY-α6 (BNAP) 2C-B-PYR 2CBecca 2CJP 2CLisaB 2CLisaH 3-Benzhydrylmorpholine 3-Phenylpiperidines (e.g., 3-phenylpiperidine , 3-PPP , OSU-6162 (PNU-96391) , LPH-5 , LPH-48 , Z3517967757 (Z7757) )6-AB AL-1095 Aminochromes (e.g., adrenochrome , adrenolutin )Benzazepines (e.g., fenoldopam , lorcaserin , SCHEMBL5334361 )Benzocyclobutenes (e.g., 2CBCB-NBOMe , bromotomscaline, S33005 , TCB-2 , tomscaline )Benzoxepins (e.g., BBOX, IBOX, TFMBOX )Butyltolylquinuclidine Camfetamine Cypenamine (trans -2-phenylcyclopentylamine) Diphenidine Diphenylprolinol DMBMPP Ergolines (e.g., LSD )Fencamfamin GYKI-52895 HDMP-29
Ivabradine Methoxphenidine Methylmorphenate
Milnacipran MT-45 2-Naphthylamine Org 6582 Partial ergolines (e.g., NDTDI , RU-27849 , DEIMDHPCA , DEMPDHPCA , DEMPDHPCA-2C-D , RU-27251 )PF-592,379 Phenylcyclopropylamines (e.g., DMCPA , TMT , tranylcypromine )Phenylpiracetams (e.g., phenylpiracetam , MRZ-9547 , RGPU-95 )Pyridopyrroloquinoxalines (e.g., lumateperone , deulumateperone , IHCH-7079 , IHCH-7086 , IHCH-7113 , ITI-1549 )Tetrahydrobenzopyranylamines (e.g., CT-5126 )Tolazoline Tricyclics (e.g., AMDA , AMDH, benzoctamine , dizocilpine , SpAMDA)ZC-B
Related compounds
2-Furylethylamine 2-Pyrrolylethylamine 3-Pyrrolylethylamine 3-Pyrrolylpropylamine 2-Tetrahydrofurylethylamine 4-Benzylpiperidine 7-AB Alkylamines (e.g., 1,3-DMBA Tooltip 1,3-dimethylbutylamine , 1,4-DMAA Tooltip 1,4-dimethylamylamine , heptaminol , iproheptine , isometheptene , methylhexanamine/1,3-DMAA , octodrine , oenethyl , tuaminoheptane )Benzylamines (e.g., benzylamine , α-methylbenzylamine , MDM1EA , ALPHA , M-ALPHA , pargyline )Benzylpiperazines (e.g., benzylpiperazine , MDBZP , fipexide )Cyclohexylaminopropanes (e.g., propylhexedrine , norpropylhexedrine )Cyclopentylaminopropanes (e.g., isocyclamine , cyclopentamine )Phenoxyethylamines (e.g., 3,4,5-trimethoxyphenoxyethylamine, CT-4719 , ORG-37684 )Phenylalkenylamines (e.g., phenylbutenamine )Phenylalkynylamines (e.g., phenylbutynamine )Phenylpiperazines (e.g., 1-phenylpiperazine , mCPP Tooltip meta-chlorophenylpiperazine , TFMPP Tooltip trifluoromethylphenylpiperazine , oMPP Tooltip ortho-methylphenylpiperazine , pFPP Tooltip para-fluorophenylpiperazine , pMeOPP Tooltip para-methoxyphenylpiperazine )Phenylpropylamines (e.g., phenylpropylamine , homo-MDA , homo-MDMA )Thienylaminopropanes (thiopropamines) (e.g., thiopropamine , methiopropamine , thiothinone )
See also: TryptaminesErgolines and lysergamides Stimulants Entactogens Psychedelics