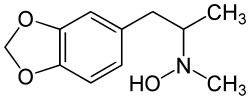
3,4-Methylenedioxy-N-hydroxy-N-methylamphetamine
 | |
| Clinical data | |
|---|---|
| Other names | 3,4-Methylenedioxy-N-hydroxy-N-methylamphetamine; 3,4-Methylenedioxy-N-methyl-N-hydroxyamphetamine; MDMOH; MDHMA; N-Hydroxy-MDMA; FLEA |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H15NO3 |
| Molar mass | 209.245 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
3,4-Methylenedioxy-N-hydroxy-N-methylamphetamine, also known as MDMOH, MDHMA, or FLEA, is an entactogen, psychedelic, and stimulant of the phenethylamine, amphetamine, and MDxx families. It is the N-hydroxy homologue of MDMA ("Ecstasy"), and the N-methyl homologue of MDOH. FLEA was first synthesized and assayed by Alexander Shulgin.[1] In his book PiHKAL (Phenethylamines i Have Known And Loved), Shulgin listed the dosage range as 100–160 mg, and the duration as approximately 4–8 hours.[1] He describes FLEA as causing entactogenic and open MDMA-like effects, easing communication, and increasing appreciation of the senses.[1] Shulgin explained the reasoning for naming the compound "FLEA" in PiHKAL.[1]
Legality
United Kingdom
This substance is a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act.[2]
See also
References
- ^ a b c d Shulgin A, Shulgin A (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ^ "UK Misuse of Drugs act 2001 Amendment summary". Isomer Design. Archived from the original on 22 October 2017. Retrieved 12 March 2014.
External links
| Tryptamines |
| ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenethylamines |
| ||||||||||||||||||||||
| Lysergamides |
| ||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||
| Natural sources |
| ||||||||||||||||||||||
| |||||||||||||||||||||||
| DRAsTooltip Dopamine releasing agents |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAsTooltip Norepinephrine releasing agents |
| ||||||||||||||
| SRAsTooltip Serotonin releasing agents |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||