Levisoprenaline
 | |
| Clinical data | |
|---|---|
| Other names | l-Isoprenaline; l-Isoproterenol; (-)-Isoproterenol; (R)-Isoprenaline; (-)-Isoprenaline; (R)-Isoproterenol; L-(-)-Isoproterenol |
| Drug class | Sympathomimetic; β-Adrenergic receptor agonist; Bronchodilator |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.161.507 |
| Chemical and physical data | |
| Formula | C11H17NO3 |
| Molar mass | 211.261 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
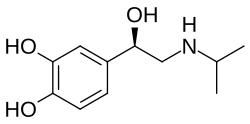
Levisoprenaline (INNTooltip International Nonproprietary Name) is a sympathomimetic, β-adrenergic receptor agonist, and bronchodilator which was never marketed.[1][2][3] It is the levorotatory or (R)-enantiomer of isoprenaline.[3]
References
- ^ Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 710. ISBN 978-1-4757-2085-3. Retrieved 27 February 2025.
- ^ Buckingham J (1996). Dictionary of Organic Compounds. Chapman & Hall. p. 3994. ISBN 978-0-412-54090-5. Retrieved 27 February 2025.
- ^ a b Morton IK, Hall JM (2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Netherlands. p. 164. ISBN 978-94-011-4439-1. Retrieved 27 February 2025.
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.