Norbudrine
 | |
| Clinical data | |
|---|---|
| Other names | Norbutrine; RD-9338; N-Cyclobutylnoradrenaline; N-Cyclobutylnorepinephrine |
| Drug class | Sympathomimetic; Bronchodilator |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H17NO3 |
| Molar mass | 223.272 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
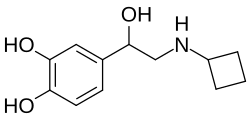
Norbudrine (INNTooltip International Nonproprietary Name; developmental code name RD-9338), also known as norbutrine (BANTooltip British Approved Name) or as N-cyclobutylnoradrenaline, is a drug of the phenethylamine and catecholamine families described as a sympathomimetic and bronchodilator which was never marketed.[1][2][3] It is the N-cyclobutyl analogue of norepinephrine (noradrenaline).[1][4] The drug was first described in the literature by 1966.[1]
References
- ^ a b c Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 1-PA215. ISBN 978-1-4757-2085-3. Retrieved 17 October 2024.
- ^ List PH, Hörhammer L (2013). Chemikalien und Drogen Teil A: N-Q. Handbuch der Pharmazeutischen Praxis - Vollständige (4.) Neuausgabe (in German). Springer Berlin Heidelberg. pp. 265–266. ISBN 978-3-642-65035-2. Retrieved 17 October 2024.
- ^ "-drine sympathomimetics" (PDF). The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2018 (Stem Book 2018). World Health Organization.
- ^ "Norbudrine". PubChem. U.S. National Library of Medicine. Retrieved 17 October 2024.
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.