Salmefamol
 | |
| Clinical data | |
|---|---|
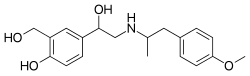
| Other names | AH-3923; AHR-3929; 1-(4-hydroxy-3-(hydroxymethyl)phenyl)-2-(4-methoxy-α-methylphenethylamino)ethanol |
| Routes of administration | Inhalation, intravenous[1] |
| Drug class | Bronchodilator; β-Adrenergic receptor agonist |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H25NO4 |
| Molar mass | 331.412 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Salmefamol (INNTooltip International Nonproprietary Name, BANTooltip British Approved Name; developmental code name AH-3923) is a drug of the phenethylamine and amphetamine families described as a bronchodilator which was never marketed.[2] It is a β-adrenergic receptor agonist with some selectivity for the β2-adrenergic receptor[3][4] and has been described as a "sister compound" to salbutamol.[5] However, the drug is more potent (1.5-fold), longer-acting (6 hours), and more lipophilic in comparison to salbutamol.[6][1] It was intended for inhalational or intravenous administration.[1] Salmefamol was first described in the literature by 1968.[2]
References
- ^ a b c Patrick GL (2017). An Introduction to Medicinal Chemistry. Oxford University Press. p. 665. ISBN 978-0-19-874969-1. Retrieved 17 October 2024.
- ^ a b Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 1089. ISBN 978-1-4757-2085-3. Retrieved 17 October 2024.
- ^ Labrid C, Rocher I, Guery O (November 1989). "Structure-activity relationships as a response to the pharmacological differences in beta-receptor ligands". American Journal of Hypertension. 2 (11 Pt 2): 245S – 251S. doi:10.1093/ajh/2.11.245s. PMID 2573372.
- ^ Leclerc G, Rouot B, Velly J, Schwartz J (1981). "β-Adrenergic receptor subtypes". Trends in Pharmacological Sciences. 2. Elsevier BV: 18–20. doi:10.1016/0165-6147(81)90248-0. ISSN 0165-6147.
- ^ Clayden J, Greeves N, Warren S (2012). Organic Chemistry. OUP Oxford. p. 530. ISBN 978-0-19-927029-3. Retrieved 17 October 2024.
- ^ Kummer F (2012). Treatment of Asthma: The long-acting beta-2-agonists. Springer Vienna. p. 38. ISBN 978-3-7091-7513-2. Retrieved 17 October 2024.
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.